Neat Tips About How To Become A Notified Body

Lists of notified bodies can.
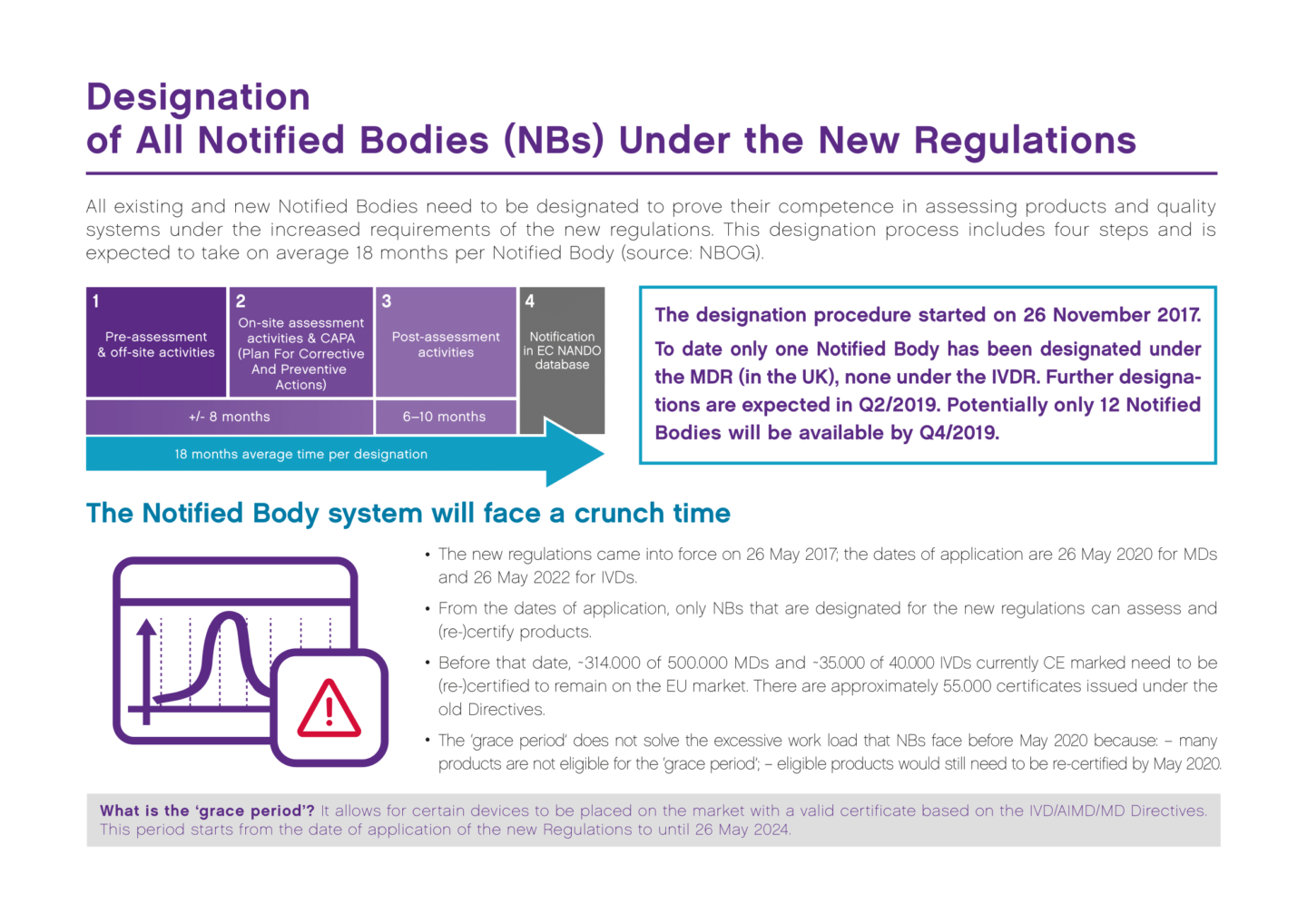
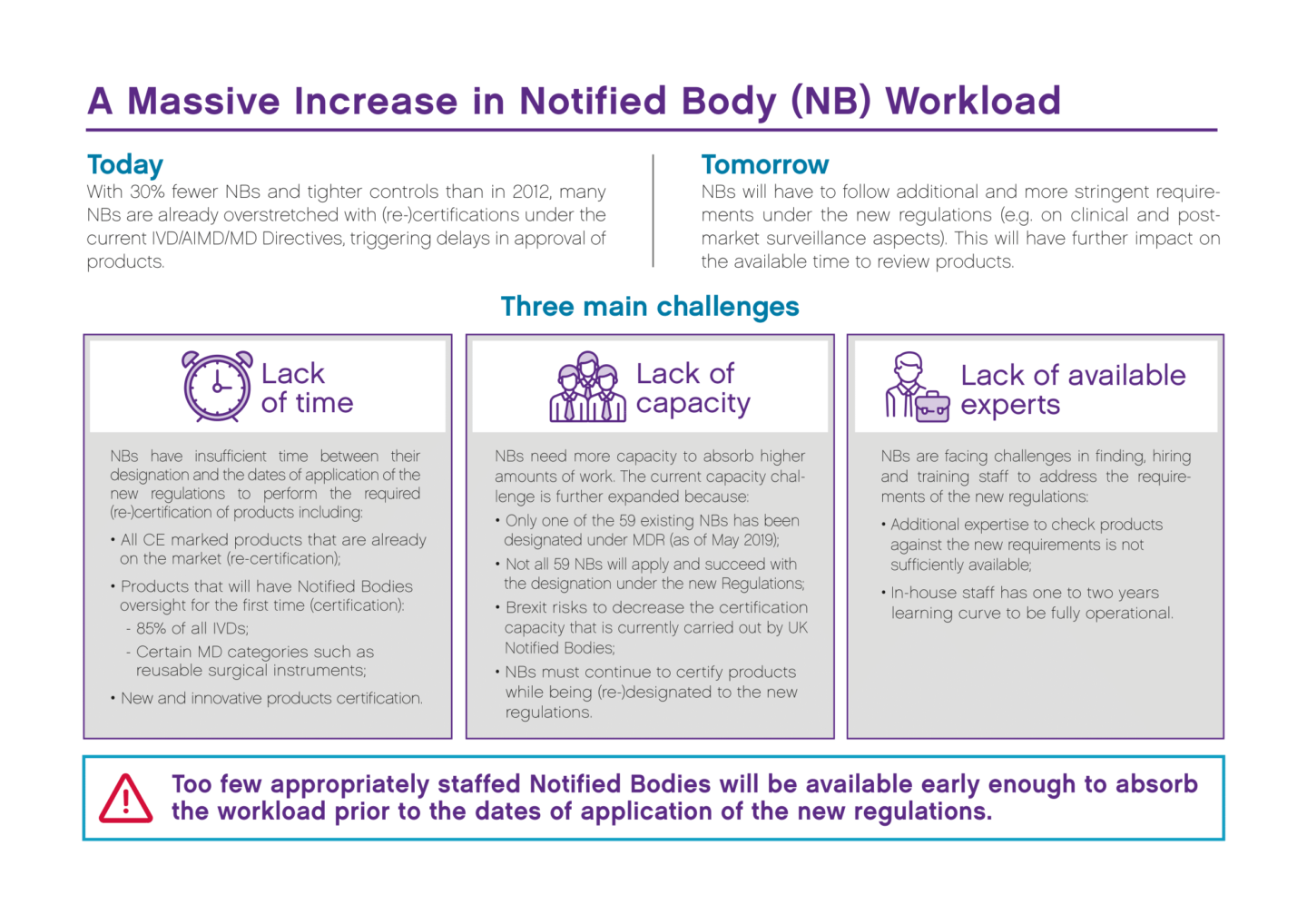
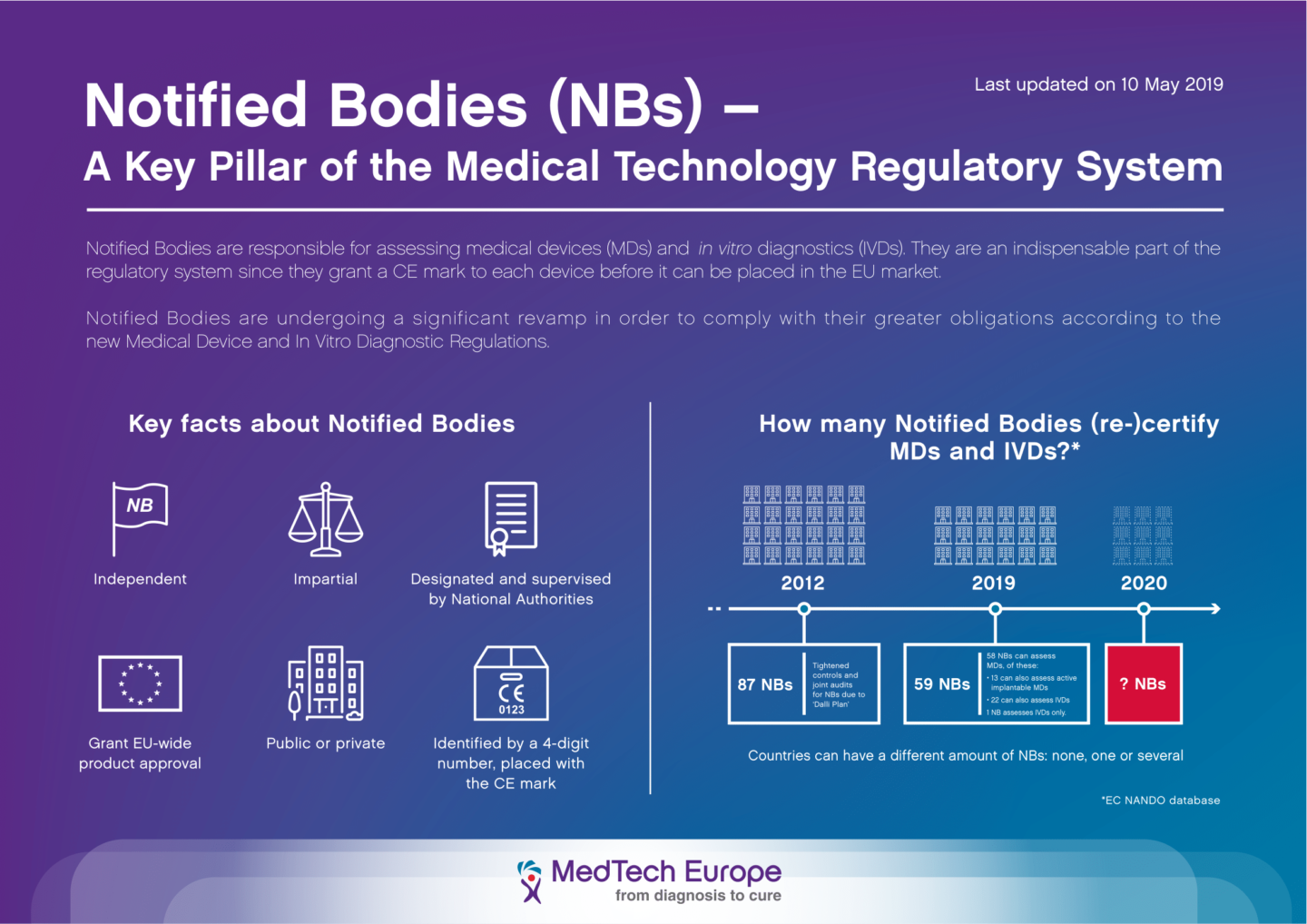
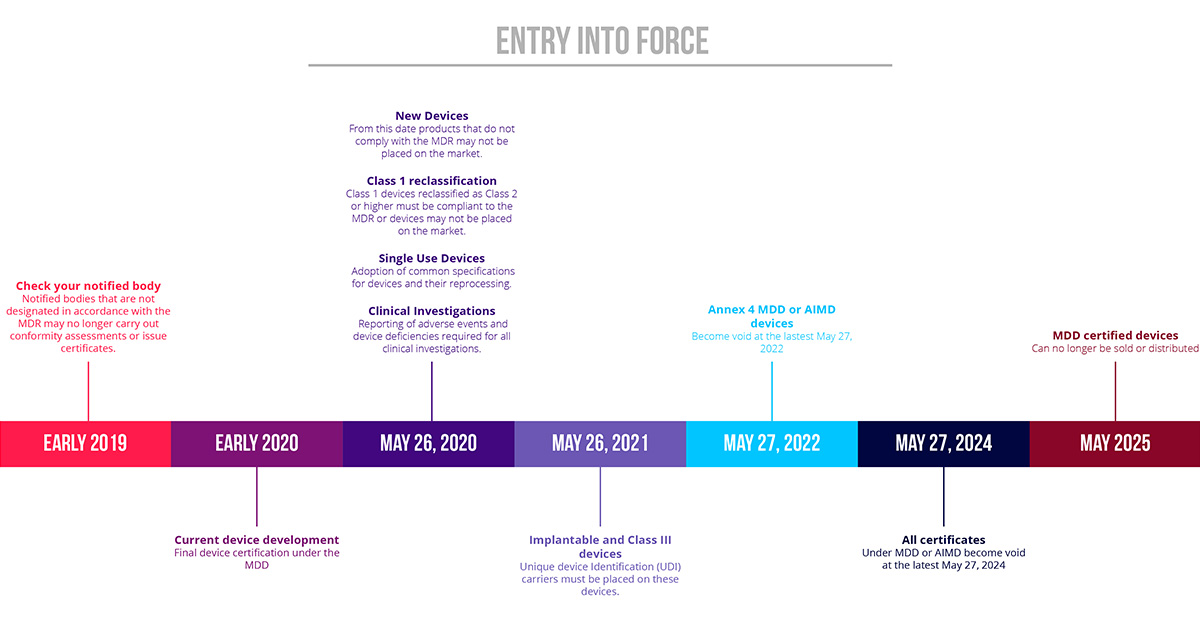
How to become a notified body. Your notified body audits your. Our registrar of iso9000 and iso13485 is also our notified body for ce. The solution is to test it by performing some simulation.
Information to an entity that wishes to become a notified body an entity that wishes to be appointed as notified body must apply for accreditation for the purpose of notifications using. This webinar continues a series of webinars on amendments in construction legislation discussing a very important topic for all conformity assessment bodies (certification bodies. Become a pefc notified certification body.
And for all but the lowest risk devices. If you are a certification body looking to offer pefc certification to forest owners and/or companies in the supply chain you need to take care of. My company manufactures class iia medical devices following annex ii of the mdd.
Within three years from publication (i.e. A notified body, in the european union, is an organisation that has been designated by a member state to assess the conformity of certain products, before being placed on the eu market,. You simulate that you have received some complaints and that you are going through them.
Discover the steps and the career path to progress in your career as a notified body medical. Before you can market your product in the european union, you’ll need a ce marking for your device.







![Requirements Relating To Notified Bodies For Eu Mdr [Video] - Learngxp: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/04/ELM-320-01-Requirements-Relating-to-Notified-Bodies-for-EU-MDR.png)